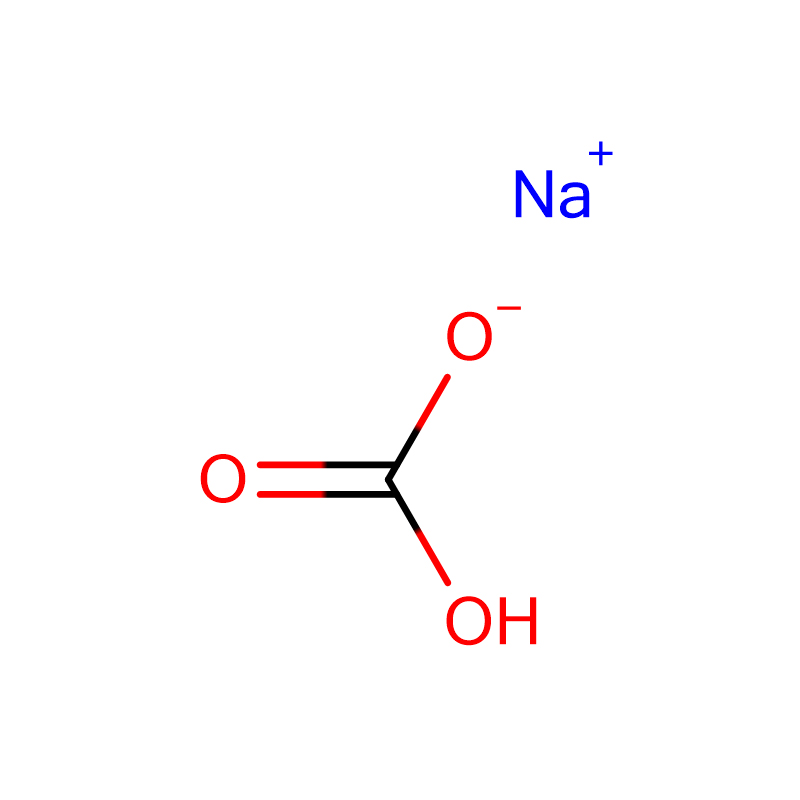
Sodium bicarbonate Cas: 144-55-8
| Nọmba katalọgụ | XD91855 |
| Aha ngwaahịa | sodium bicarbonate |
| CAS | 144-55-8 |
| Ụdị molekụlala | CHNaO3 |
| Ibu molekụla | 84.01 |
| Nkọwa nchekwa | 2-8 Celsius C |
| Koodu tarifu akwadoro | 28363000 |
Nkọwapụta ngwaahịa
| Ọdịdị | Ọcha ntụ ntụ |
| Asay | 99% nkeji |
| Ebe mgbaze | > 300 Celsius C (ọkụ ọkụ) |
| Ebe esi nri | 851°C |
| njupụta | 2.16 g/mL na 25 Celsius C (ọkụ) |
| index refractive | 1.500 |
| solubility | H2O: 1M na 20 Celsius C, doro anya, enweghị agba |
| Nnukwu ike ndọda | 2.159 |
| Isi | Enweghị isi |
| PH | 8.3 (0.1 edoziri nke ọma) |
| Ọnụ ego PH | 7.8-8.2 |
| pka | (1) 6.37, (2) 10.25 (carbonic (na 25 ℃) |
| Mmiri Solubility | 9 g/100 ml (20ºC) |
| Nbibi | 50 Celsius C |
Sodium bicarbonate, nke a na-eji n'ụdị soda na ntụ ntụ, bụ ihe na-eko achịcha na-emekarị.Mgbe a na-agbakwunye soda soda, nke bụ ihe alkaline, na ngwakọta, ọ na-eji ihe acid eme ihe iji mepụta carbon dioxide.Enwere ike ịnọchite anya mmeghachi omume dị ka: NaHCO3(s) + H+ → Na+(aq) + H2O(l) +CO2(g), ebe acid na-eweta H+.Nri ntụ ntụ nwere soda soda dị ka ihe bụ isi yana acid na ihe ndị ọzọ.Dabere na nhazi ahụ, ndị na-eme achịcha nwere ike ịmepụta carbon dioxide ngwa ngwa dị ka otu ntụ ntụ ntụ ntụ ma ọ bụ na nkebi, dị ka ọ na-eme ntụ ntụ.A na-ejikwa soda soda eme ihe dị ka isi iyi carbon dioxide maka ihe ọṅụṅụ carbonated yana dịka ihe nchekwa. Na mgbakwunye na ịsa mmiri, soda nwere ọtụtụ ihe eji eme ụlọ.A na-eji ya dị ka ihe mkpocha izugbe, deodorizer, antacid, ọkụ na-ekpo ọkụ, na ngwaahịa onwe onye dị ka ntacha eze. Njirimara, nke pụtara na ọ nwere ike rụọ ọrụ dị ka ma ọ bụ acidor ntọala.Nke a na-enye soda baking ikike ịgha mkpụrụ na ike iwepụ ma ntọala acid na ntọala.Enwere ike wepụ isi nri sitere na acidic ma ọ bụ ogige ndị bụ isi na bakingsoda n'ime nnu na-enweghị isi.N'ihi na sodium bicarbonate bụ isi adịghị ike, ọ nwere ikike ka ukwuu iji wepụ isi acid.
Ojiji nke abụọ kachasị ukwuu nke sodium bicarbonate, na-aza ihe dịka 25% nke mkpokọta mmepụta, bụ ihe mgbakwunye nri ọrụ ugbo.Na anụ ụlọ ọ na-enyere aka ịnọgide na-enwe rumen pH ma na-enyere fiber digestibility;maka anụ ọkụkọ ọ na-enyere aka ịnọgide na-enwe nguzozi electrolyte site n'inye sodiumin nri, na-enyere anụ ufe aka ịnagide okpomọkụ, ma na-eme ka akwa akwa akwa.
A na-eji sodium bicarbonate eme ihe na ụlọ ọrụ kemịkalụ dị ka onye na-eme ihe na-eme ka ọ na-eme ihe, ihe na-agba agba, ihe na-akpali akpali, na nri nri.A na-eji sodium bicarbonate mee ihe na akpụkpọ anụ akpụkpọ anụ maka ịkwado na ihicha mkpuchi na ijikwa pH n'oge usoro tanning. Sodium bicarbonate na-emepụta sodium carbonate, nke a na-eji ncha na iko. Ering agent, na n'ụdị dị ka isi iyi nke carbon dioxide na eff ervescent mbadamba.Ụdị ọkụ ọkụ na-ekpo ọkụ BC na-emenyụ ọkụ nwere sodium bicarbonate (ma ọ bụ potassium bicarbonate) .Ojiji ndị ọzọ a na-eji bicarbonate gụnyere nhazi nke pulp na akwụkwọ, ọgwụgwọ mmiri, na mmanụ olulu mmiri.
Sodium Bicarbonate bụ ihe na-eko achịcha nwere ph nke ihe dịka 8.5 na ngwọta 1% na 25ºc.ọ na-arụ ọrụ na nri ọkwa phosphates (ihe na-eko achịcha acidic) iji hapụ carbon dioxide nke na-agbasa n'oge a na-eme achịcha iji nye ihe ndị a esiri esi na ụbara olu na àgwà iri dị nro.a na-ejikwa ya na ihe ọṅụṅụ na-ekpo ọkụ na-ekpo ọkụ iji nweta carbonation, nke na-eme mgbe a na-agbakwunye mmiri na ngwakọta nke nwere sodium bicarbonate na acid.ọ bụ akụkụ nke ntụ ntụ ntụ.a na-akpọkwa ya baking soda, bicarbonate of soda, sodium acid carbonate, na sodium hydrogen carbonate.
imepụta ọtụtụ nnu sodium;isi iyi nke CO2;mgwa ihe nke ntụ ntụ, nnu na-esi ísì ụtọ na ihe ọṅụṅụ;na ihe ọkụ ọkụ, ihicha Ngwakọta.
sodium bicarbonate (baking soda) bụ nnu inorganic a na-eji dị ka onye na-ebufe ihe na onye na-edozi pH, ọ na-arụkwa ọrụ dị ka onye na-ekpo ọkụ.A na-eji ya na ntụ ntụ na-eme ka akpụkpọ ahụ dị nro.